UX Audit
UX Research
Usability Testing
FDA Formative & Summative Testing
Title 21 FDA regulations
UX Design
UX Architecture
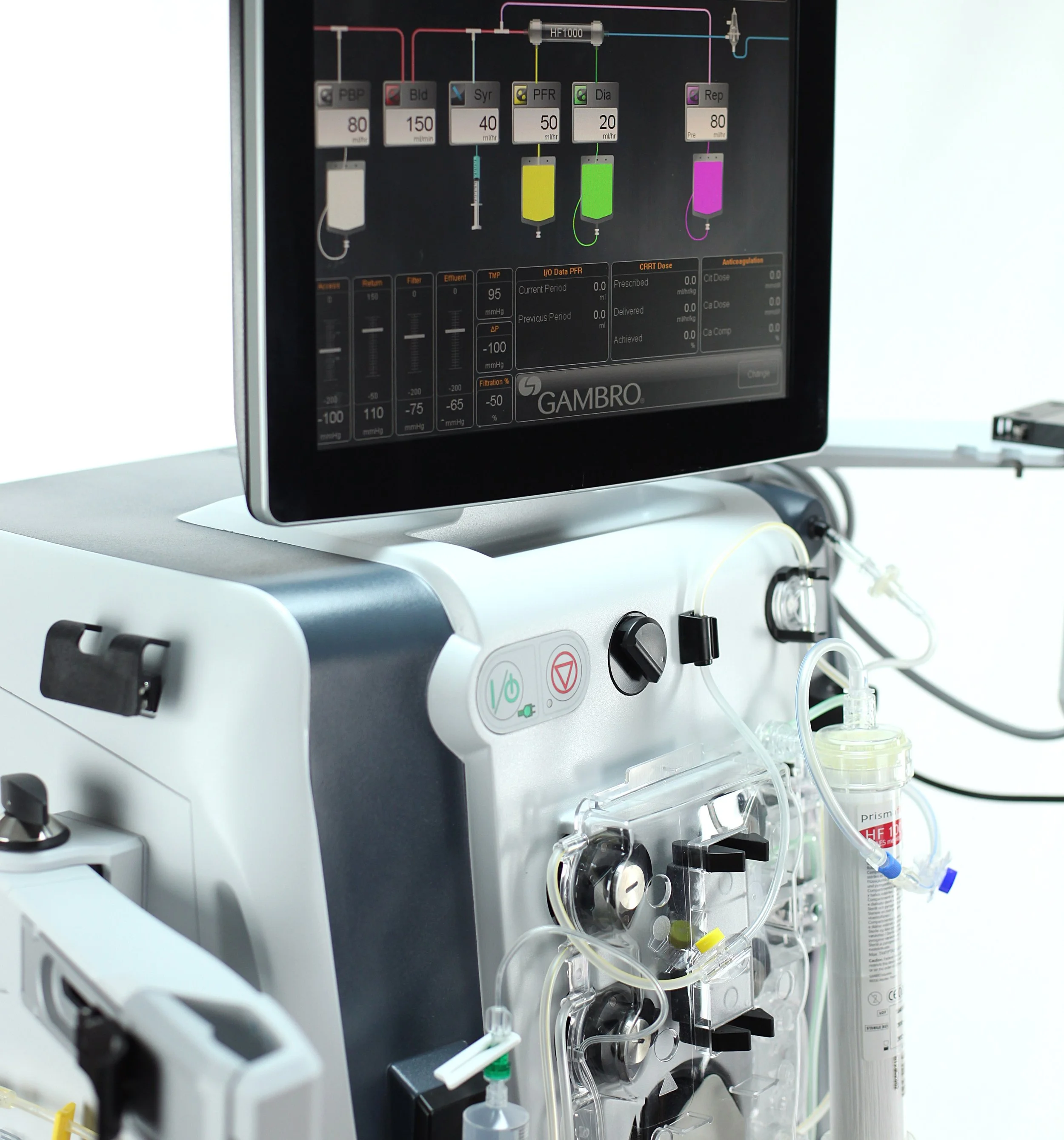
REVE partnered with Baxter to design the user experience for the PrisMax, an advanced medical device used in continuous renal replacement therapy (CRRT) for critically ill patients. Our team immersed itself in the complex world of dialysis to deeply understand the workflows, pressures, and human factors within the ICU environment. Through extensive contextual inquiry and observation of clinicians in real-world settings, we uncovered the challenges associated with fluid management, blood anticoagulation, and continuous therapy delivery—areas where precision, safety, and clarity are paramount.
Designing for such a mission-critical context required us to create an embedded user interface that was not only intuitive and efficient, but also met the demanding constraints of a regulated medical device platform. We developed a design system that prioritized clarity, error prevention, and streamlined task flows, helping clinicians focus on patient care rather than device navigation. REVE’s team worked closely with engineers and clinical experts to translate complex therapy protocols into a visual and interaction language that reduced cognitive load and supported confident decision-making, even under pressure.
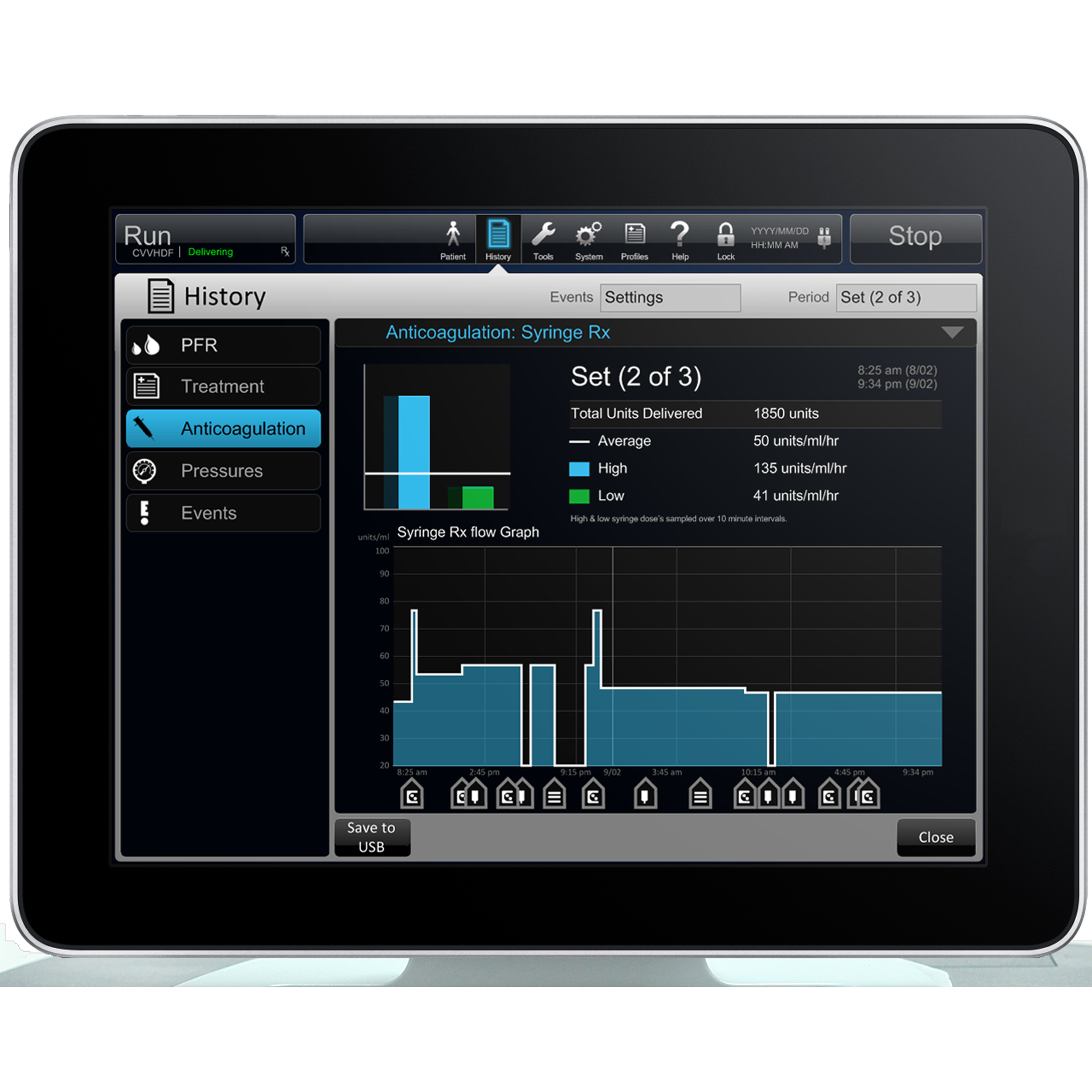
Throughout the process, REVE conducted multiple rounds of Formative and Summative usability testing to validate design decisions and ensure compliance with FDA and IEC 62366 usability standards. These sessions with real ICU nurses and nephrologists directly shaped the evolution of the interface, from wireframes to production-ready specifications. The resulting experience not only redefined usability expectations in the dialysis category but also led to several awarded patents, reflecting REVE’s innovative approach to workflow simplification, information hierarchy, and safety-critical design.